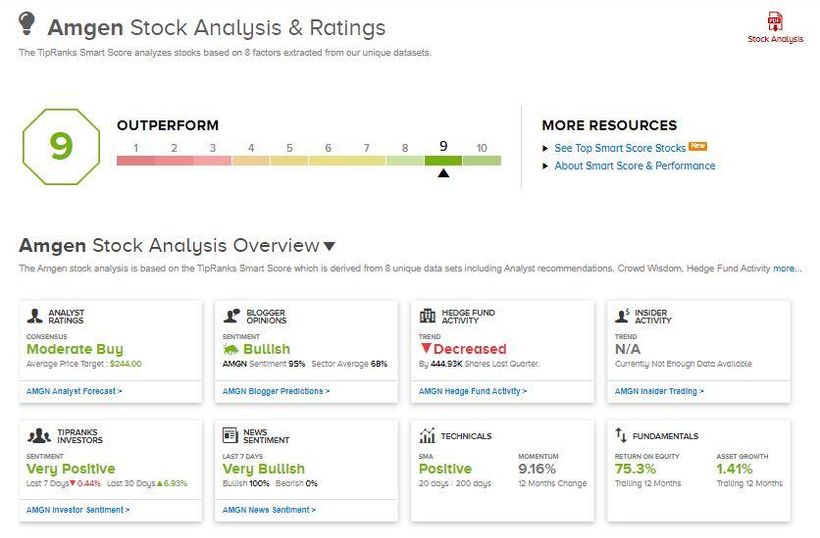
The FDA has given Amgen (AMGN) the green light to bring its breakthrough lung cancer drug Lumakras to the market. Amgen is a global biotechnology company that focuses on developing solutions to address unmet medical needs.
Lumakras won accelerated regulatory approval to treat KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC) in adults. The FDA approval was based on the outcome of the CodeBreak 100 clinical trial program.
Of the 2.2 million new lung cancer cases…